Skin disinfection – mucosal and wound antisepsis
The skin: protection from the outside
In preventive medicine, skin and hygiene are inextricably linked. Skin has many functions, from protecting and sensing, and absorbing and storing nutrients to regulating temperature and eliminating toxins. Above all, however, it is the largest sensory organ and vital protective shield against infectious pathogens. However, when attacked or wounded, its protective function rapidly deteriorates. It is important to prevent unnecessary stress on the skin at an early stage.
Skin plays a decisive role in effective hygiene management. If it is intact, it protects us from any invasive microorganisms. Damaged and wounded skin, on the other hand, is an open door for bacteria, fungi and viruses. If they enter the body, they can have serious health consequences.
At the same time, the skin itself is often a carrier of infections and can potentially contaminate patients and staff with pathogens.
When it comes to skin antisepsis, indications are decisive in choosing the respective procedure. If you want to prepare a patient for surgery or a central venous catheter, the antiseptics used should be more intensive than is the case with a puncture, e.g., of a peripheral vessel.
(Pre-)Cleansing of the Skin
(Pre-)cleansing of the skin
Skin disinfection prior to surgery, joint puncture and CVC
In human medicine, it is recommended that patients wash thoroughly the evening before the procedure or directly on the day of the procedure to achieve a significant reduction in skin flora. [1, 2]
As this is difficult to implement for most animal patients, thorough cleaning of the operating area after shearing should be carried out.
The aim is to reduce transient skin flora and to remove sebum, grease, and dirt. Do not use soap or water as this can reduce the effect of the subsequent antiseptic (alcohol). [3]
Skin can be cleansed with a dry foam or cleansing lotion.
Apply the product to the skin with a low-germ compress or cloth and clean the skin thoroughly. After 1-2 minutes, dry with a clean cloth or swab.
For home washing by the owner, the patient’s fur should be thoroughly rubbed and cleaned with a washcloth soaked in cleansing solution. After 3-5 minutes, rub the fur dry with a clean towel.
Skin Disinfection
Skin disinfection
Effective protection for patients and staff
Skin antiseptics plays a key role in the prevention of SSIs, as the transection of the skin allows any resident flora to enter deeply into the operated on area.
Due to its rapid and good efficacy, alcohol is one of the first choices. [1, 2] The presence of residual active ingredients has been a matter of some debate.
In some countries, PVP-iodine or chlorhexidine is traditionally the agent of choice. There is some debate from human medical literature as to which active ingredient should be favored or indeed avoided due to negative effects.
In a meta-analyses of the different studies, neither of the two antiseptics were found to be superior over the other. [2, 4]
However, the reduced effect of chlorhexidine towards multi-resistant Staphylococcus aureus compared to PVP-iodine has been pointed out. [5]
Other authors report chlorhexidine resistance in Escherichia coli, Salmonella spp., Staphylococcus aureus or coagulase negative staphylococci, Enterobacter spp., Pseudomonas spp., Proteus spp., Providencia spp. and Enterococcus spp. [6]
We therefore recommend the use of an alcohol PVP-iodine preparation (Braunoderm®).
The patient’s skin is rubbed for 30 seconds with a sterile swab soaked in Braunoderm® along with the aid of grain forceps. In this case, work is always carried out from the intended resection area outwards. The contact time of the manufacturer is then observed and the process is repeated two more times.
PVC iodine (Braunol®) or polyhexanide can be considered for mucosal antisepsis. [1]
Skin disinfection prior to puncturing
The skin should be adequately sheared. Disinfection is divided into two phases: In the first step, the skin is wetted with an alcohol preparation (Softasept® N) and wiped with a swab after the respective contact time. The skin is then wetted again with the disinfectant and the contact time is waited out for before the puncture is carried out.
Alternatively, wipe the skin with a swab soaked in alcohol (Softasept ® N). This cleanses the skin of dirt and sebum and achieves a mechanical reduction of germs. In the second phase, the skin is wetted again and the contact time is waited out for before the puncture is carried out.
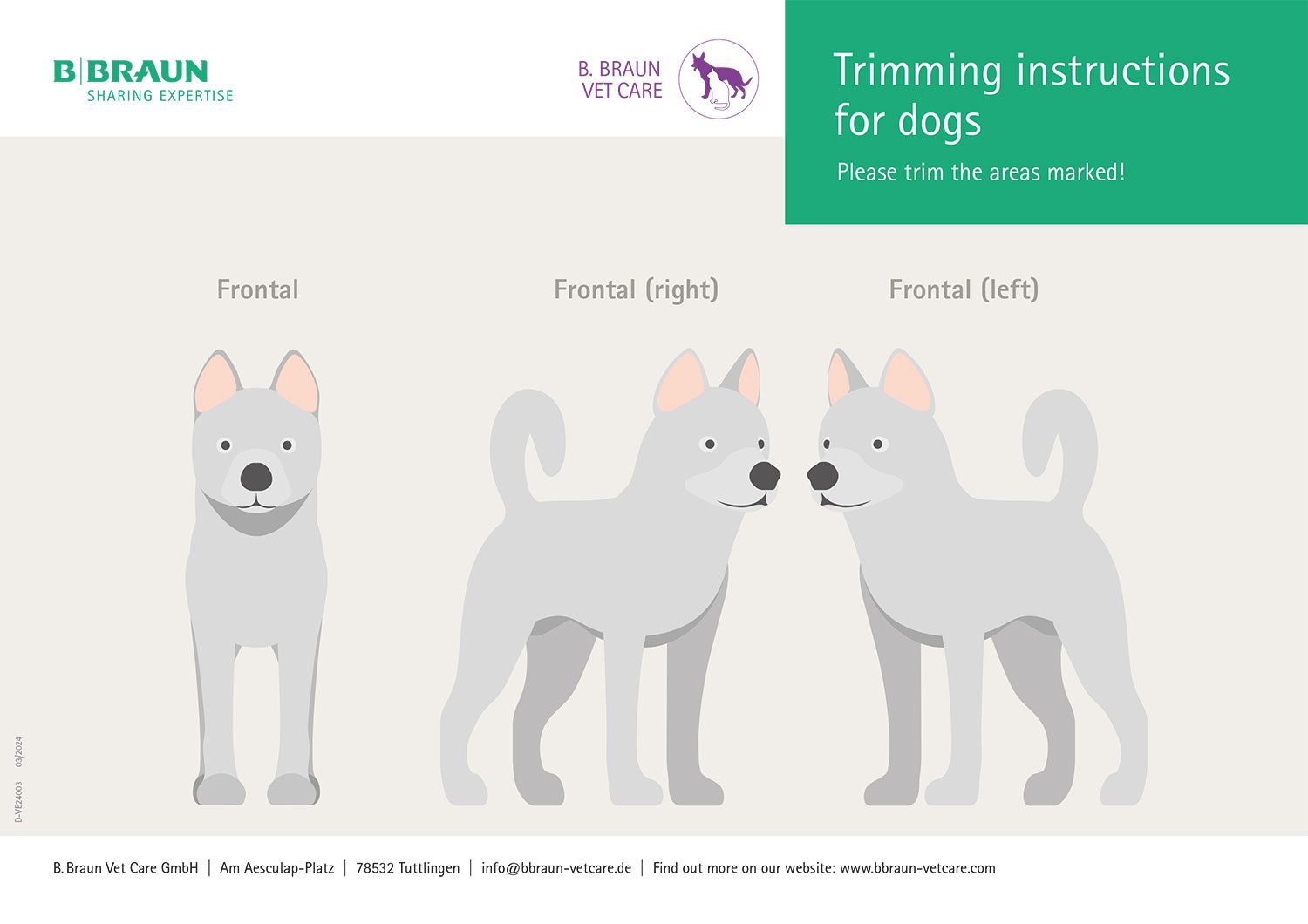
Step-by-step guide
Alcohol-based skin antiseptic containing iodine (Braunoderm®)
Wipe disinfection
Step-by-step guide
Alcohol-based skin antiseptic containing iodine (Braunoderm®)
Spray disinfection
Every second counts: contact times for skin disinfection
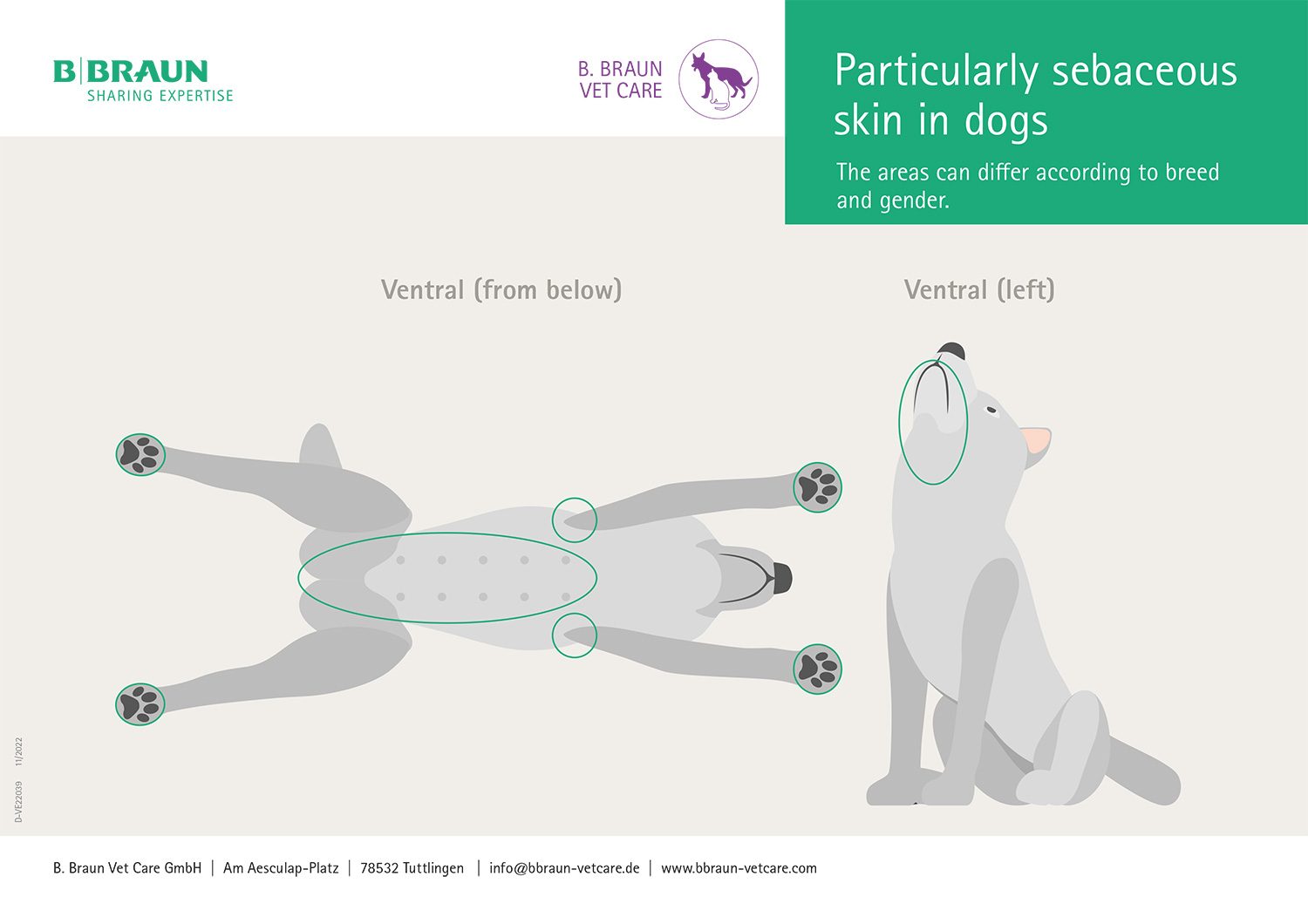
Skin has a large surface area, yet the individual regions of the body differ significantly from one another much like a geographical map. The sebaceous gland density of a specific area of the body is decisive for the required contact time for skin disinfection. The largest density of sebaceous glands in dogs and cats can be found, for example, in the areas under the limbs and the groin area.
Disinfection of skin areas rich in sebaceous glands requires longer contact times than disinfection of areas poor in sebaceous glands. Here, the bacterial count is significantly higher and resident microorganisms predominate.
The contact times for the individual medical procedures are just as different: a simple needle suture requires shorter skin disinfection times than an invasive surgical procedure. Both factors are decisive for determining the contact time of a skin disinfection measure.
The respective contact times vary depending on the preparation so that the corresponding manufacturer’s specifications should always be referred to for this purpose.
Graph
Particularly sebaceous skin in dogs
The areas can differ according to breed and gender.
Graph
Mucosal Antisepsis
Mucosal antisepsis
Sensitive task with high value
The mucosa differs from normal skin in its structure and therefore requires different steps for an effective disinfection. The microbial colonization of the mucosa is influenced by various factors including the animal species, breed, age, hormonal influences, sex, and pathological factors.
Under normal circumstances, the resident flora of the mucosa represents a stable microbial ecosystem in which various aerobic and anaerobic microorganisms reside. If this system is disrupted by endogenous or exogenous factors, the mucosa can be increasingly colonized by undesirable microorganisms.
In the case of an endogenous infection, it should be noted that the microorganisms found in the mucosa can be actively or passively introduced into originally germ-free body areas. The use of medical devices, such as cystoscopes, places mechanical strain on the mucosa and is a potential cause of the spread of germs in hollow organs. However, these devices can also contribute to exogenous infections if they are not properly disinfected.
The reduction of microorganisms on the mucosa and adjacent areas ensures the prevention of endogenous and exogenous infections. However, mucous membranes place high demands on an antiseptic: in addition to the need to act microbicidally against transient and resident flora, the skin tolerance of the mucosa is of particular importance.
The alcohol compounds usually used for normal skin disinfection lead to pain reactions and local intolerances for mucosal antisepsis and should therefore not be used. The choice and use of an antiseptic is therefore not only based on medical requirements. The topographical circumstances of the mucous membrane are also relevant.
Preventative use
The aim of a prophylactic application is to minimize the risk of endogenous and exogenous infections before diagnostic, therapeutic and nursing measures are initiated. A broad, rapid, and sustained efficacy of an antiseptic is just as important as any local and systemic tolerability.
Therapeutic indications
The therapeutic use of antiseptics allows systemic antibiotics to be refrained from and is intended to alleviate or heal any diagnosed local infections.
Products for mucosal antisepsis
Wound Antisepsis
Wound antisepsis
Which active ingredients are suitable?
The relevant antiseptic agents are polyhexanide (PHMB), iodophors, octenidine (OCT), sodium hydrochloride/hydrochloric acids (NaOCl/HOCl) and silver ion formulations. [7, 8] As antimicrobial efficacy and cell toxicity are directly proportional to each other, good tolerability is often achieved at the expense of efficacy. Other factors such as active substance concentration, duration of exposure, type of application, and the microenvironment of the wound should also be taken into account when selecting an antiseptic.
Polyhexanide solutions at a concentration of 0.02 percent are suitable for the preventive reduction of a microbial pathogen burden. Polyhexanide solution at a concentration of 0.04-0.1 percent (ProntoVet® Solution) is recommended for infected wounds. [7, 8]
Iodophors (PVP iodine) are the first choice for deep bite and stab wounds. [7, 8]
PVP iodine applications
While the focus in aqueous and alcoholic PVP iodine solutions is on achieving rapid effects, the long-lasting effects on wounds are key when it comes to ointments with 10 percent PVP-iodine.
Braunol® with 7.5 percent PVP iodine (povidone-iodine) has proven itself as an aqueous antiseptic both in concentrated and diluted form for decades.
In ophthalmology, aqueous PVP iodine solutions have been successfully used in dilutions from 1:3 to 1:6.
PVP-iodine can also be used as a ready-to-use, alcoholic solution such as Braunoderm® for rapid disinfection prior to punctures and surgical procedures.
Braunovidon® ointment with 10 percent PVP-iodine is ideally suited for the external therapy of burns and scalding, and treatment of abrasion, laceration, and abscess wounds. [7]
Step-by-step guide
Downloads Skin Antisepsis
Skin antisepsis
[1] Prevention of surgical site infections. Bundesgesundheitsbl 2018; 61(4):448-73.
[2] Maiwald M, Widmer AF. WHO's recommendation for surgical skin antisepsis is premature. Lancet Infect Dis 2017; 17(10):1023-4.
[3] Hübner N-O, Kampf G, Löffler H, Kramer A. Effect of a 1 min hand wash on the bactericidal efficacy of consecutive surgical hand disinfection with standard alcohols and on skin hydration. Int J Hyg Environ Health 2006; 209(3):285-91.
[4] Marchionatti E, Constant C, Steiner A. Preoperative skin asepsis protocols using chlorhexidine versus povidone-iodine in veterinary surgery: A systematic review and meta-analysis. Vet Surg 2022; 51(5):744-52.
[5] Block C, Robenshtok E, Simhon A, Shapiro M. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect 2000; 46(2):147-52.
[6] Kampf G. Acquired resistance to chlorhexidine – is it time to establish an 'antiseptic stewardship' initiative? J Hosp Infect 2016; 94(3):213-27.
[7] Kramer A., Daeschlein G., Kammerlander G. Konsensusempfehlung_Wundantiseptik_2003 (Consensus recommendation for wound antisepsis 2003). Zeitschrift für Wundheilung 2004; (3).
[8] Kramer A, Dissemond J, Kim S, Willy C, Mayer D, Papke R et al. Consensus on Wound Antisepsis: Update 2018. SPP 2018; 31(1):28-58.